The cave where it happened: Denisova cavern’s congress of ancient peoples
A second high-coverage Denisovan genome holds a mirror up to our own lineage
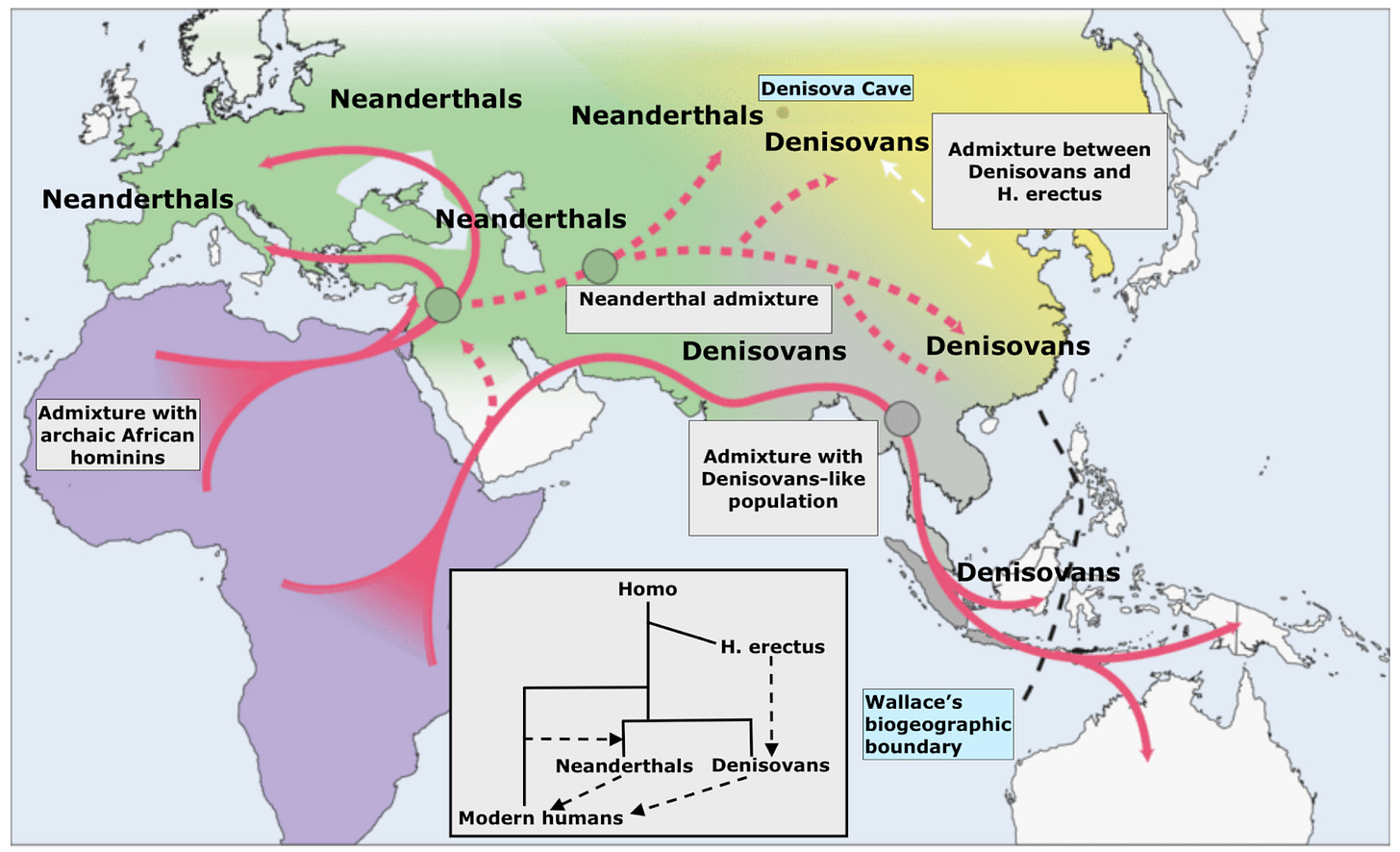
No one was looking for her or her kin when in 2010 scientists sequenced the genome of a woman who died in Siberia tens of thousands of years before modern humans arrived there. Digging into her genome, they beheld the first evidence of a whole new branch of humanity. From her genes alone, researchers identified the human population we now call Denisovans, drawing the name from that first source location, Denisova Cave, a cluster of high-vaulted caverns in central Siberia, north of the border with western Mongolia. To start, the team typed the woman’s mitochondrial DNA, tracing her direct maternal line. This was the first shock: her unknown human lineage was more distant from ours than Neanderthals are. Next, a whole-genome analysis, scanning all three billion base pairs, revealed more nuance. In fact, she was genetically more similar overall to Neanderthals than to our own out-of-Africa lineage; this realization spawned the neologism, “Neandersovans,” for the shared grouping of Neanderthals and Denisovans.
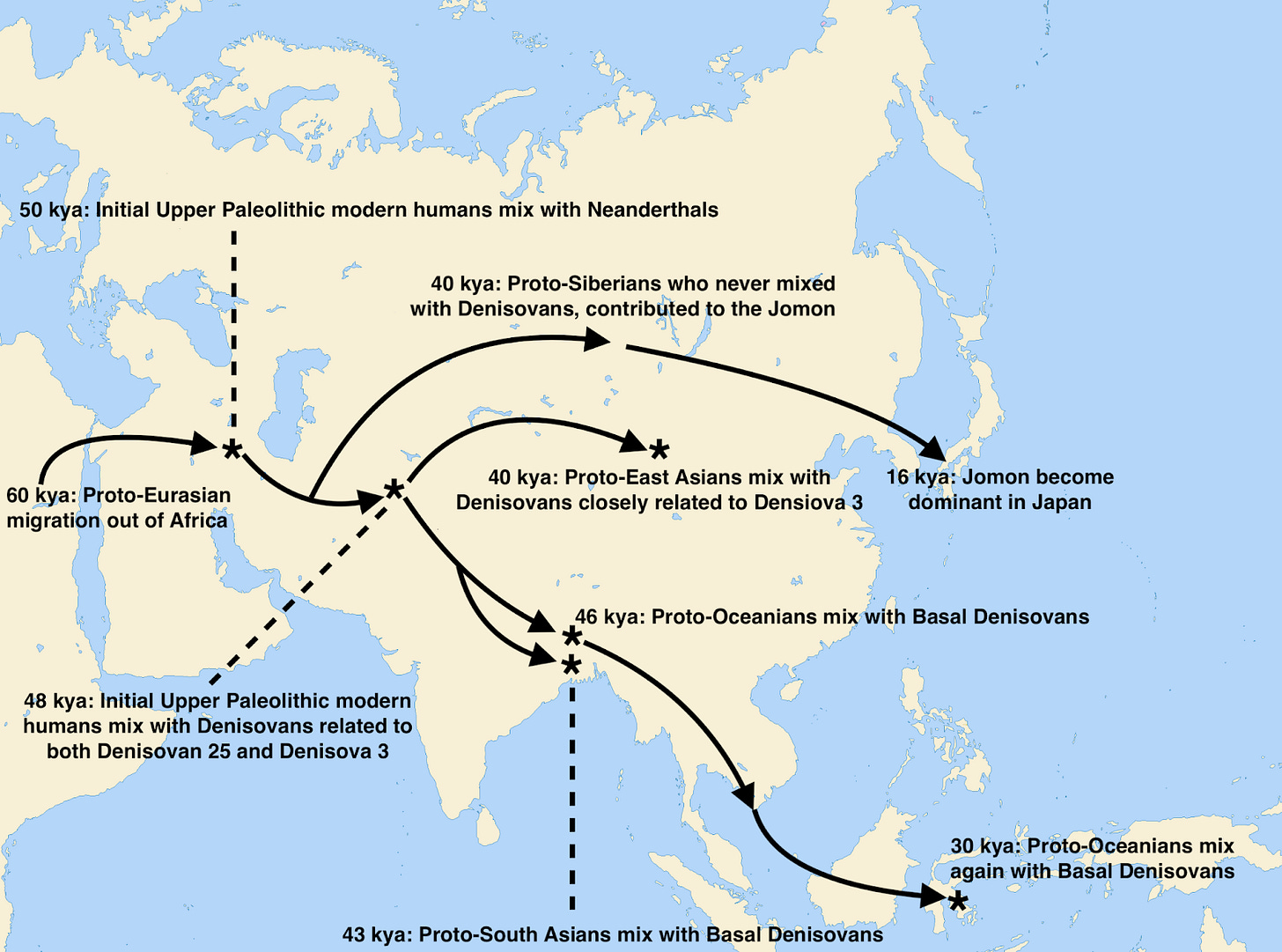
So this single genome from a woman interred in Denisova Cave brought the revelation that over 500,000 years ago a group of humans left Africa to spread out across Eurasia, trekking north and east, becoming Neanderthals and Denisovans respectively. With the abstruse arts of statistical genomics, researchers have since also inferred from that single genome that multiple admixture events with separate Denisovan lineages contributed trace ancestry to modern populations, from the Siberian Denisovans responsible for an average 0.15% of ancestry in East Asians today, to the southern Denisovans who contributed 4% of modern Papuan ancestry.
Even though a single genome is but a single human being born at a single point in time, at the molecular level, we truly cannot shake our past. The indelible ledger heredity smuggles into every genome makes any random being a decoder ring equipped to spill the population history of their species generations deep into their past. The millions of variable positions in any individual genome faithfully reflect population history, reporting highlights from an individual’s entire genealogy, allowing reconstruction of an entire vast lineage, with inferences fanning backward in an ever-branching ancestral tree.
Although we now have genetic material from many more than a single Denisovan (for example, mitochondrial DNA from 10 individuals), because all the other samples have been comparatively incomplete, for paleogeneticists the high-quality whole genome from 2010 has continued to do the load-bearing work. This is the individual we call Denisova 3, sequenced at 30-fold coverage, meaning geneticists have sampled each region of her genome about thirty times to assure themselves of the accuracy of each position’s precise read. Not only is this excellent if we want to undertake deep evolutionary genomic analysis, it is modern medicine’s gold standard for medical-grade genetic inference (insurance companies traditionally require 30-fold coverage for whole-genome tests). Despite finding further fossils, attaining what they call environmental DNA (from debris where the group lived rather than even trace identifiable human remains) and snippets of sequence here and there from other Denisovans, the bulk of our genomic understanding of these enigmatic humans over the last 15 years has remained the legacy of Denisova 3. Until today.
Finally, at the end of 2025, a preprint reports a new high-quality whole-genome sequence, at 24-fold coverage: A high-coverage genome from a 200,000-year-old Denisovan. Though researchers found this individual, Denisova 25, at the same location, he is vastly older than Denisova 3, who died 65,000 years ago. Not only does this allow geneticists to probe deeper into the past (the rough estimate is that a staggering 5,400 generations separate Denisovan 25 from Denisova 3!), it finally allows for comparative genomic analyses, apples-to-apples high-quality genome to high-quality genome.
Denisovan 25 has already yielded fresh insights, from further confirmation of the carryover of pre-Denisovan humans into their lineage (and so ultimately, into parts of ours), to refinements in the understanding of Denisovan population structure and the timing of admixtures. The sequencing of a new Denisovan might not be as field-altering as the species’ 2010 discovery, but in 2025 we can say we are truly getting to grips with this people; we can now discern how many races they subdivided into, how many distinct times they mixed with different modern human populations and even the lineaments of their mysterious interactions among even more ancient and barely understood human lineages that preceded them into Asia.
A cave where worlds collided
In science, you work with the data you can get, not the data you might want. For a host of reasons — geopolitical, archaeological and climatological — we have very little ancient DNA from Africa, despite the continent being the main stage for human evolution over the last two million years. Remote, singular Denisova Cave is at the other extreme; the single site is an embarrassment of riches. With the cavern complex’s relative surfeit of ancient Eurasian DNA, we have been able to piece together the dynamics of African evolution; never mind that Eurasian humans were not in Africa, their ancestors were, and those ancestors left their imprint on the genomes their descendants bore all across Eurasia. About a dozen humans have thus far been identified at the Denisova Cave location. The multi-chambered complex of caverns not only yielded the first Denisovan whole-genome, in 2014 researchers also found the first high-coverage Neanderthal there, known in the literature as the “Altai Neanderthal” (and which to this day, remains one of only three Neanderthals sequenced at over 20x coverage). Even more sensationally, in 2018 Denisova’s chambers yielded “Denny,” a young woman who lived 90,000 years ago, issue of a pure Neanderthal mother and Denisovan father. Finally, the caves have yielded 20,000-year-old DNA from a modern human of the “Ancient North Eurasian” (ANE) population. The characteristics that made the high-vaulted shelter a desirable home for our Eurasian cousins clearly still applied long after their disappearance and replacement by our single lineage.
Why is Denisova Cave such a fruitful hunting ground for paleoanthropology and paleogenetics? Part of the answer is clearly down to geology and climate, what ecologists would term “abiotic” parameters. For most of the last two million years the region was extremely cold and dry, with the cave surrounded by tundra steppe. And luckily for future researchers, cold, dry conditions favor the preservation of biological remains that might otherwise degrade, from DNA to coprolites. But evolutionary factors were also at play; Denisova Cave happens to straddle the extreme edges of both Neanderthal and Denisovan prehistoric ranges. For Neanderthals, it marked the eastern edge, for Denisovans it was apparently along their northwestern boundary. This means that the cave witnessed hundreds of thousands of years of congress between these two human groups, including matings recorded in the genes of the people who frequented the cave. Denny was only the clearest and most obvious case of this mixing. Denisova 25 attests not just to further instances of admixture from Neanderthals, but of other human lineages too that are yet unidentified ghosts in our understanding of ancient DNA.
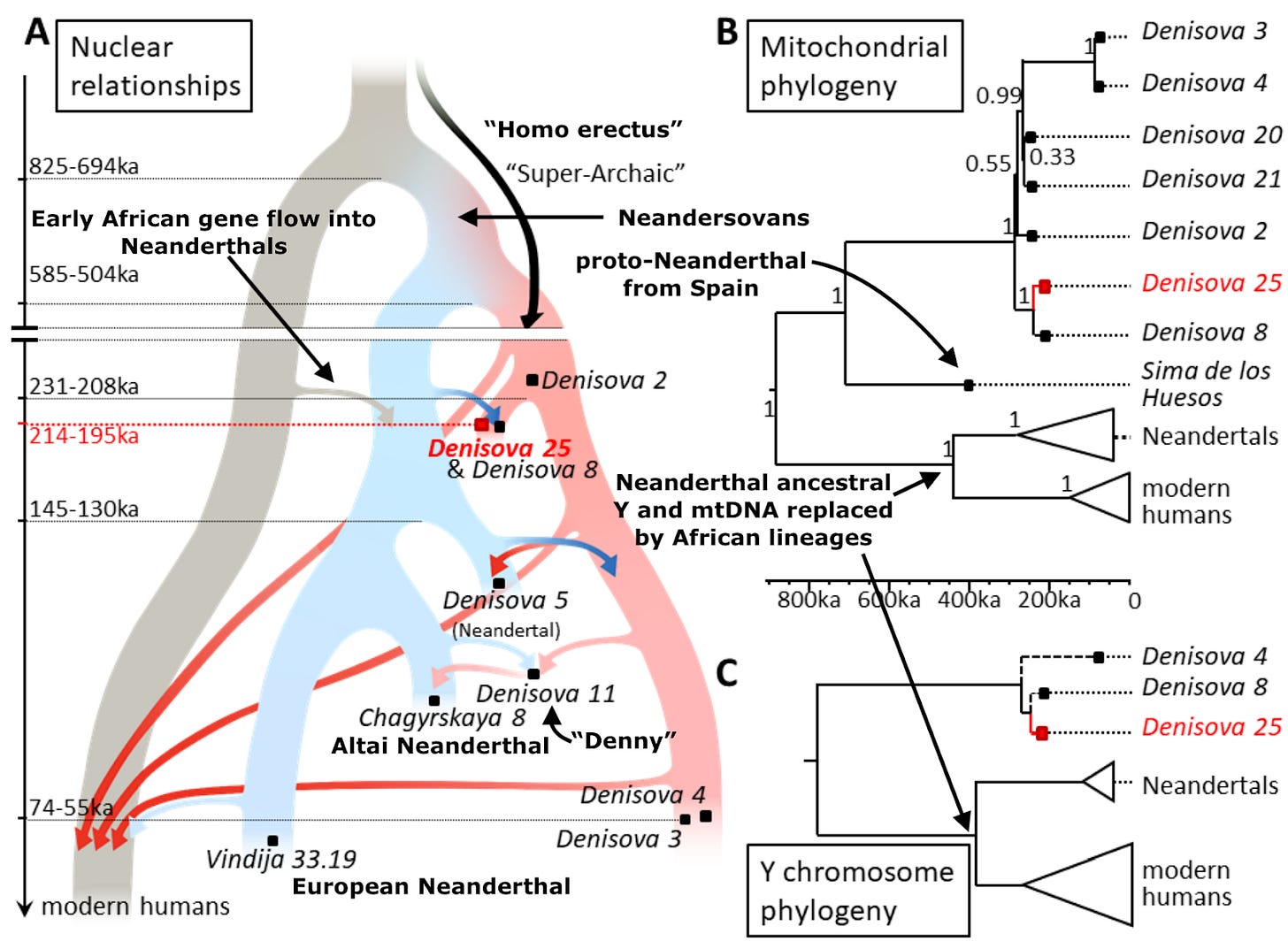
Relationships of Denisova 25 to archaic and modern humans
A quick scan of the genome reveals Densiova 25 is clearly male; he has a Y chromosome. He clusters as expected with the other Denisovan males whose paternal lineage could be typed. The mtDNA lineage also clusters with other Denisovans. You will recall that since the Y and mtDNA lineages are not subject to recombination, they have since long before the genomic age been go-to’s for simple and elegant reconstruction of phylogenetic trees. The researchers dated the sample to some 219,000-232,000 years ago by comparing the mutations that define changes between Denisova 25 and the other dated Denisovans. Happily, comparing the entire genome of Denisova 25 to modern humans (again using those clock-like assumptions about rates of mutation accreted since last common ancestor) converges on roughly the same time span.
Though Denisova 3 and Denisova 25 are the only two high-quality genomes from the caverns recovered thus far, Denisova 2, 4, 8 and 11 each yielded enough material for simple phylogenetic analyses. Comparing numbers of shared and unshared genetic variants across the genomes, Denisova 8 and Denisova 25 are particularly genetically close, unsurprising since their positions within the sedimentary layers excavated date them to similar periods. Denisova 4 and 11 are noticeably more similar to Denisova 3 than to Denisova 8; again, no big surprise when you consider the times when their position suggests they lived. The newly discovered Denisovan genome is genetically closer to Neanderthal sequences than the 65,000-year-old Denisova 3 is, reflecting that the former bears more Neanderthal ancestry. Unlike the other samples mentioned here, Denisova 5 is a Neanderthal, and not a Denisovan human. Additionally, the new Denisova 25 individual’s genes are more similar to the Denisova 5 Neanderthal’s than to all other sequenced Neanderthals. Because this Neanderthal dates to about 60,000 years after Denisova 25 lived, the most likely explanation is that the ancestors of the Neanderthal labeled Denisova 5 had absorbed Denisovan ancestry specifically from a population related to Denisova 25.
A generation ago, many scholars of human evolution remained skeptical of hybridization between our species’ various lineages. They commonly believed Neanderthals and African humans had been separated too long for interbreeding to be viable. But after the 2010 publication of the Neanderthal genome, and the finding that all non-Africans (as well as the vast majority of Africans!) have Neanderthal ancestry, all that changed. The addition of this second high-quality Denisovan genome, almost 150,000 years earlier in Denisova Cave’s eventful history, enriches our understanding of interactions between the various branches of our human family tree. Humans outside of Africa today average about 2% Neanderthal, while Denisova 3 was 1.8-2.5% and the far earlier Denisova 25 measured an estimated 3.6-5.2% Neanderthal. Additionally, Denisova 25 has Neanderthal admixture that is genetically equidistant to multiple Neanderthal genomes, indicating it derives from an unknown and yet unsampled Neanderthal lineage. Denisova 25 and 3’s high-quality genomes enable precise detection of Neanderthal ancestry segments interspersed in a sea of Denisovan ancestry. The average segment lengths, a reliable index correlating with time since mixture occurred, suggest that the Neanderthal ancestry input to both Denisova 25 and 3 arrived via several distinct events. In other words, Denisova Cave apparently played backdrop to repeated interactions between human lineages over tens of thousands of years. So now with hindsight, it isn’t entirely surprising that researchers stumbled upon Denny, a hybrid whose ancestry composition was probably far more common than originally assumed.
And yet even with samples from vastly different time periods, we identify pure Neanderthal and Denisovan individuals over and over in the cave, rather than witnessing the emergence of a new Neandersovan hybrid population. This implies that while Denisovan Cave was a meeting point, it proved more of a demographic sink than a transit junction between two populations. The hybridization events didn’t lead to enduring, discernible gene flow between Neanderthals and Denisovans as broader populations; rather, the local populations at Denisovan Cave emerged over and over again, only to go extinct repeatedly, leaving no longer-term legacy. The cave may have been optimal for the preservation of DNA, but in the depths of the ice age it was clearly a refuge in an incredibly harsh local ecology.
Among the most exciting upgrades granted us by a second high-quality genome is the chance to more fully delve into the possibility of “super-archaic” admixture in the broader Denisovan lineage. We want to settle whether Denisovans, whose ancestors arrived in eastern Eurasia less than a million years ago, might have mixed there with various earlier arrived human lineages. Think of the peoples who in an earlier era of scholarship were bracketed under the label Homo erectus. The earliest attested Homo outside of Africa seems to be fossils of the lineage labeled Homo georgicus at the Dmanisi site in Georgia 1.8 million years ago, and in short order, that hominin’s relatives spread eastward all the way to the Pacific. Meanwhile, most paleoanthropologists believe that human groups like the Flores hobbits and the diminutive Luzon hominins did not descend from the Neandersovan wave that arrived less than a million years ago, which suggests that they too would have predated Denisovans’ tenure, underscoring that prehistoric Asia was already quite speciose when the Denisovans’ ancestors arrived.
From a genomic perspective, it was clear from Denisova 3’s genome that compared to any Neanderthal, she shared fewer novel mutations with African humans and us, their modern descendants. Two possible models could explain this. One is gene flow between Neanderthals and our ancient Africans ancestors prior to 200,000 years ago, which would have cranked up the number of shared variants between Neanderthals and modern humans. The other alternative is that at some point Denisovans alone mixed with even more distantly related humans, super-archaics, genetically tugging them away from both Neanderthals and modern humans. The former hypothesis does have some support, the evidence from Y, mtDNA and whole genomes does indicate some ancient gene flow from our lineage into Neanderthals. But not enough to explain the entire extent of the genetic cleavage between our two groups and Denisovans.